Does Co2 Have Dipole Dipole Interactions
Since both are gases at room temperature they do not interact with each other. Int J Chem Kinetics 19.
B dispersion forces and ion-dipole C dispersion forces and dipole-dipole D dispersion forces ion-dipole and dipole-dipole E None.
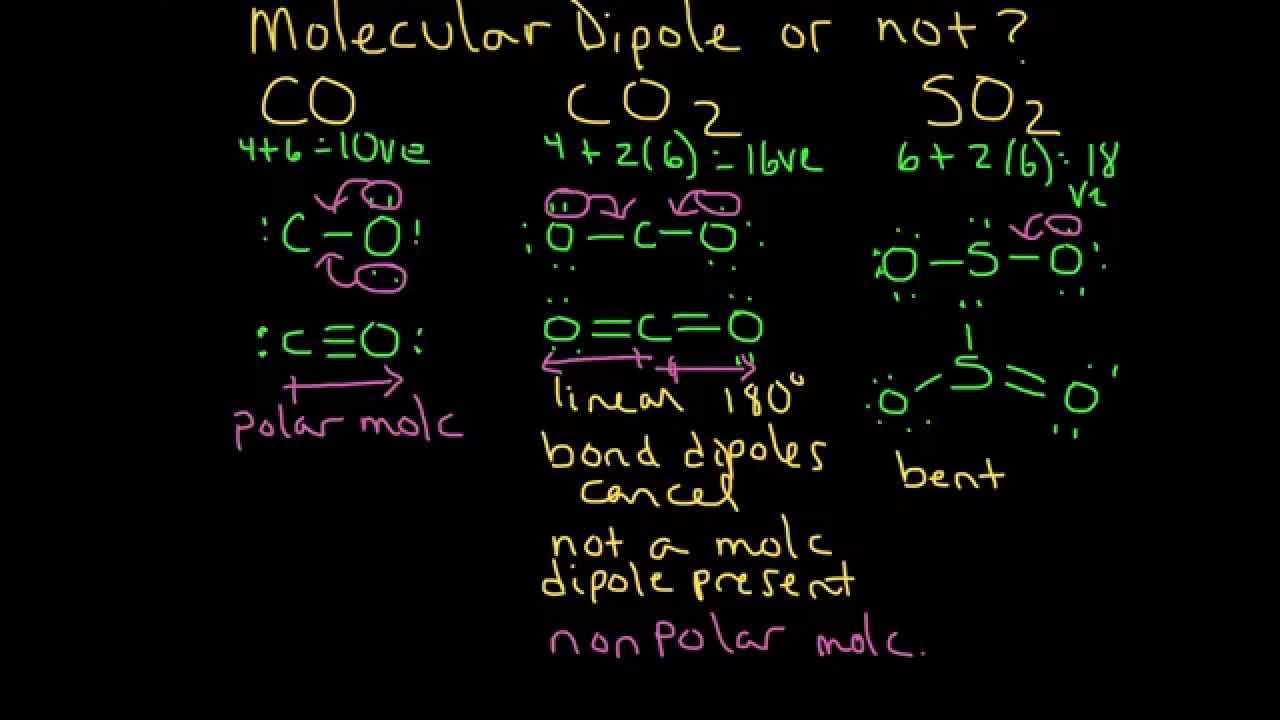
. The nonpolar liquid will have the higher boiling point since the polar. The polar liquid will have the higher boiling point since its molecules have dipole-dipole interactions. Further Ga does not follow the periodic table product trend and has by others been proven to produce formic acid in oxide form 32 and are thus omitted in this work.
Dry ice is the solid form of carbon dioxideIt is commonly used as it does not have a liquid state and sublimates directly from the solid state to the gas stateIt is used primarily as a cooling agent but is also used in fog machines at theatres for dramatic effects. Why does CO2 not have a permanent dipole. Because methane is a non-polar molecule it is not capable of hydrogen bonding.
Hazardous Substances Data Bank HSDB Hydroxy radical rate constant 74X10-11 cu cmmolecule-sec at 25 C. Moreover we note that Cd and Zn are metals that have the lowest faradaic efficiency for their main product and Cu is the only metal to go beyond CO. They will have similar boiling points since the dispersion forces depend upon molar mass.
Dipole moment at 25 C. Therefore in the case of CO2 to net dipole moment is zero and the molecule is nonpolar even though each bond is polar. The solution of allosteric coefficients with only one Bohr variable is suggested by a heterotropic Bohr equation which clarifies the biophysical symmetry in allostery.
The FE toward C 2 H 4 was 10 for current densities in the range 300 to 800 mAcm 2 fig. Dielectric constant at 25 C. Merck and Co Inc 2006 p.
The Merck Index - An Encyclopedia of Chemicals Drugs and Biologicals. Its advantages include lower temperature than that of water ice and not leaving any residue other than incidental frost from. The product selectivity shift was attributed to electrostatic interactions of cation species eg K with the electric dipole of specific adsorbates that favor C 2 reaction pathways 31 35.
The nonpolar liquid will have the higher boiling point since its molecules are more loosely held together. The net dipole moment for a molecule is the vector sum of the individual bond dipoles. Is ch4 a dipole-dipole force.
Bohr Effect Mechanism Allosteric interactions. The allosteric Bohr effects should have been chosen by nature to adjust haemoglobin to cope with the quick dynamics of gas.
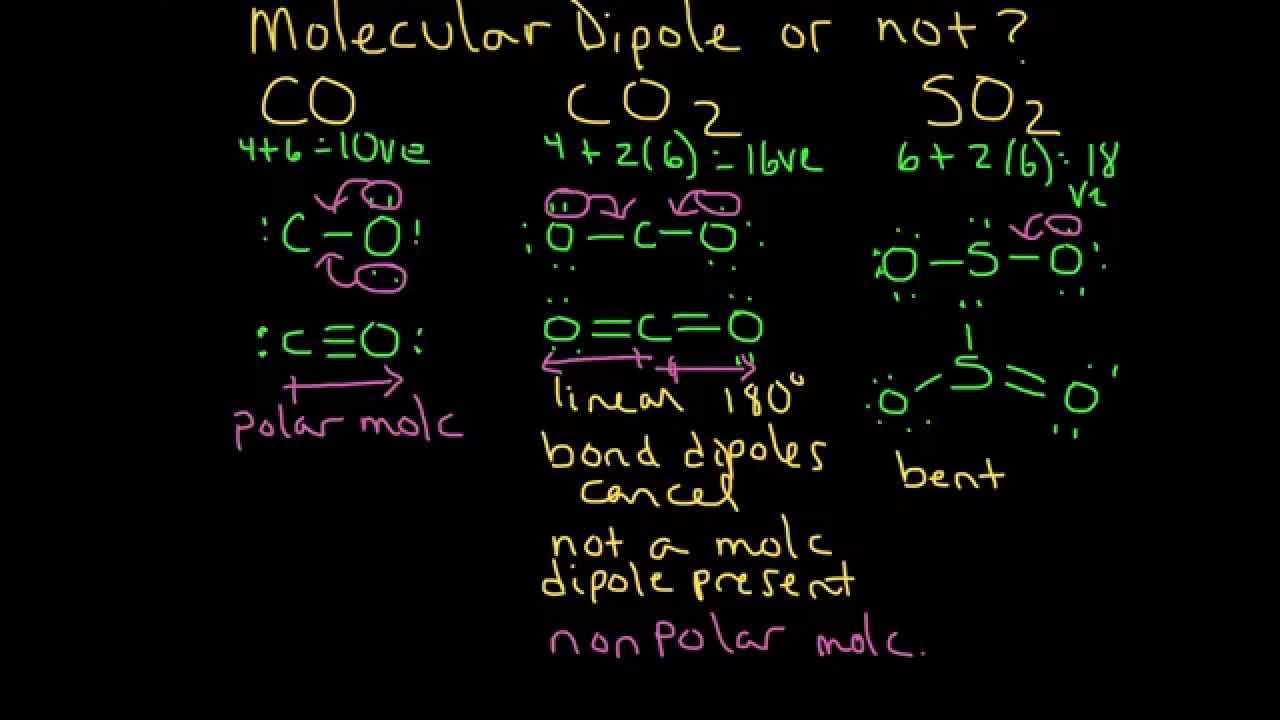
Molecular Dipole Moment Example 1 Co Co2 And So2 Youtube

Co2 Intermolecular Forces Type Strong Or Weak Techiescientist

Co2 Intermolecular Forces Type Strong Or Weak Techiescientist

Co2 Intermolecular Forces Type Strong Or Weak Techiescientist
Comments
Post a Comment